NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced the launch of the next generation of its ultrasound pain management device, PainShield Plus® for the treatment of multiple areas of pain in tandem.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201016005064/en/
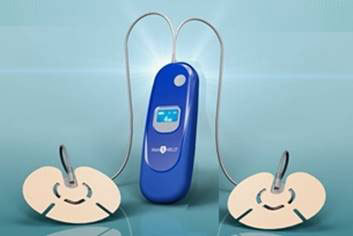
The New, Patent Pending PainShield Plus
Brian Murphy, CEO of NanoVibronix, stated, “The introduction of PainShield Plus is a natural extension of our highly effective PainShield device. Increasingly, patients are seeking therapies that work synergistically with more conventional treatment plans and reduce the need for pharmaceuticals and surgical interventions. We have extended the functionality of our core PainShield device to include an additional transducer that enables patients to cover twice the surface area for pain treatment and thereby broadens the opportunities for application of this proven technology. Initial responses from orthopedic and neurological patients and healthcare providers have been overwhelmingly positive, and we are encouraged by the prospects for wider distribution and use.”
The new, patent pending PainShield Plus includes a second adhesive patch and transducer, which effectively doubles the surface area that can be treated for pain in the same amount of time. Each transducer covers approximately 20 cm2 of surface area and with two points of access, bilateral treatment of orthopedic pain is now possible with just one device.
Like the original, PainShield Plus utilizes ultrasound therapy for the treatment of pain and various soft tissue injuries either directly over joints or orthopedic hardware and without the need for messy ultrasound gels. Importantly, the device is an effective solution for avoiding opioid treatments and supports social distancing by equipping patients to receive therapy independently in the comfort and safety of their own homes.
PainShield is an ultrasound device, consisting of a reusable driver unit and disposables, which includes a proprietary therapeutic transducer and cover adhesive. PainShield delivers a localized ultrasound effect to treat pain and induce soft tissue healing in a targeted area, while keeping the level of ultrasound energy at a safe and consistent level. Its range of applications includes acute and chronic pain resolution through its many mechanisms of action. PainShield can be used by patients at home or work or in a clinical setting and can be used even while the patient is sleeping. Patient benefits include ease of application and use, faster recovery time, high compliance, and increased safety and efficacy over existing devices that rely on higher-frequency ultrasound.
About NanoVibronix, Inc.
NanoVibronix, Inc. (NASDAQ: NAOV) is a medical device company headquartered in Elmsford, New York, with research and development in Nesher, Israel, focused on developing medical devices utilizing its patented low intensity surface acoustic wave (SAW) technology. The proprietary technology allows for the creation of low-frequency ultrasound waves that can be utilized for a variety of medical applications, including for disruption of biofilms and bacterial colonization, as well as for pain relief. The devices can be administered at home without the assistance of medical professionals. The Company’s primary products include PainShield®, UroShield® and WoundShield®, all of which are portable devices suitable for administration at home without assistance of medical professionals. Additional information about NanoVibronix is available at: www.nanovibronix.com.
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified, and include, among others, statements regarding the completion of the public offering, the satisfaction of customary closing conditions related to the public offering and the intended use of net proceeds from the public offering; consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with: (i) the geographic, social and economic impact of COVID-19 on the Company’s ability to conduct its business and raise capital in the future when needed, (ii) market acceptance of our existing and new products or lengthy product delays in key markets; (iii) negative or unreliable clinical trial results; (iv) inability to secure regulatory approvals for the sale of our products; (v) intense competition in the medical device industry from much larger, multinational companies,; (vi) product liability claims; (vii) product malfunctions; (viii) our limited manufacturing capabilities and reliance on subcontractor assistance; (ix) insufficient or inadequate reimbursements by governmental and/or other third party payers for our products; (x) our ability to successfully obtain and maintain intellectual property protection covering our products; (xi) legislative or regulatory reform impacting the healthcare system in the U.S. or in foreign jurisdictions; (xii) our reliance on single suppliers for certain product components, (xiii) the need to raise additional capital to meet our future business requirements and obligations, given the fact that such capital may not be available, or may be costly, dilutive or difficult to obtain; (xiv) our conducting business in foreign jurisdictions exposing us to additional challenges, such as, e.g., foreign currency exchange rate fluctuations, logistical and communications challenges, the burden and cost of compliance with foreign laws, and political and/or economic instabilities in specific jurisdictions; and (xv) market and other conditions. More detailed information about the Company and the risk factors that may affect the realization of forward looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at: http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events, or otherwise, except as required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20201016005064/en/
Contacts:
NanoVibronix, Inc.
bmurphy@nanovibronix.com
(630) 338-5022
Or:
Brett Maas, Managing Principal, Hayden IR, LLC
brett@haydenir.com
(646) 536-7331














