
Please replace the caption for release dated October 25, 2019 with the accompanying corrected caption.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191025005191/en/
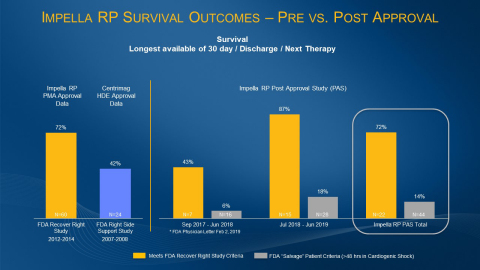
Nearly two years of real-world outcomes data on Abiomed’s Impella RP heart pump shows that when physicians followed the FDA’s approved protocol for Impella RP use they achieved 72% patient survival and 88% native heart recovery. These results, from the Impella RP’s post-approval study, match the survival rate in the Impella RP’s pre-approval study. (Graphic: Business Wire)
The release reads:
FDA POST APPROVAL STUDY DEMONSTRATES TIMELY IDENTIFICATION OF RIGHT HEART FAILURE AND EARLY USE OF IMPELLA RP LEADS TO HIGHER SURVIVAL
Nearly two years of real-world outcomes data on Abiomed’s (NASDAQ: ABMD) Impella RP heart pump shows that when physicians followed the FDA’s approved protocol for Impella RP use they achieved 72% patient survival and 88% native heart recovery. These results, from the Impella RP’s post-approval study, match the survival rate in the Impella RP’s pre-approval study. The protocol includes recommendations on patient selection and timely identification of right heart failure with subsequent implantation of Impella RP. The new data is detailed in the accompanying chart and is being presented today at a clinical meeting in Detroit for the management of patients in cardiogenic shock, led by the National Cardiogenic Shock Initiative (NCSI) Study principal investigators.
“When right-sided heart failure is identified early, it is reversable and heart recovery is possible,” said Navin Kapur, MD, the executive director of the CardioVascular Center for Research and Innovation (CVCRI) at Tufts Medical Center and principal investigator for the Cardiogenic Shock Working Group (CSWG). “Elevated RA pressure in a patient with cardiogenic shock is a warning sign that a patient is at an increased risk of dying and should trigger a comprehensive evaluation for underlying causes including right heart failure. If right heart dysfunction is identified, therapy should be escalated to manage this issue as soon as possible.”
Investigators and thought leaders in the field of cardiogenic shock have made multiple recommendations based on data sets which demonstrate late identification of right heart failure is associated with increased mortality:
- The Cardiogenic Shock Working Group found cardiogenic shock patients with elevated right arterial pressure (RAP) are at a higher risk of mortality1 and should be evaluated for RV failure as soon as possible
- The National Cardiogenic Shock Initiative Study includes the following escalation protocol: After Impella-supported PCI, and while the patient is in the catherization lab, if the patient is in right ventricular (RV) failure, quickly escalate to Impella RP2
- The cVAD Study has demonstrated patients with a left-side Impella who have a RAP or central venous pressure (CVP) of 12 mmHg or above are at higher risk of mortality (p=0.035), and rapid assessment for RV failure is recommended3
“We are pleased to see the numerical increase in survival for both the Recover Right and salvage populations in the Impella RP post approval study. The FDA’s letters to health care providers earlier this year have helped Abiomed and the post-approval study principal investigators educate other medical providers on the importance of using simple identifiers to achieve timely identification of right side heart failure in the catheterization lab and ICU,” said Seth Bilazarian, MD, the chief medical officer of Abiomed.
The FDA has confirmed the classification of Impella RP post-approval study patients into two categories: Recover Right protocol and salvage support. The Recover Right category includes patients who met the inclusion and exclusion criteria of the Recover Right FDA PMA clinical trial for Impella RP. The FDA recognizes salvage patients as those outside the Recover Right protocol (including >48 hours in cardiogenic shock from right side failure.) The baseline characteristics of the two populations are different with higher mortality in the salvage group. Salvage patients are some of the sickest patients in the hospital and many have suffered out-of-hospital cardiac arrest or may have been transferred to multiple hospitals before receiving Impella RP. The FDA and Abiomed believe that physicians should have the ability to attempt lifesaving recovery measures on these salvage patients based on their best judgement but emphasize that earlier recognition and treatment is associated with markedly better survival.
In a letter to health care providers on May 21, the FDA noted the importance of proper patient selection and timing of Impella RP insertion. The FDA wrote, “Patients who would not have qualified for the premarket clinical studies were more likely to have been in cardiogenic shock for longer than 48 hours, experienced a cardiac arrest, or suffered a pre-implant hypoxic or ischemic neurologic event before getting the Impella RP system implanted compared to the PAS patients who would have met the enrollment criteria for the premarket clinical studies.”
The FDA also wrote, “Additionally, be aware that there are currently no other device interventions that have been approved by the FDA under the premarket application (PMA) process for the patient population demonstrating a higher mortality rate in the PAS. As such, other interventions may include their own benefits and risks that should be considered and discussed with patients and their caregivers.”
Abiomed is committed to improving patient outcomes by performing FDA studies and post-market surveillance, collecting real-world evidence, and identifying and sharing best practices. Abiomed tracks outcomes on nearly 100% of its U.S. patients through its Impella Quality (IQ) Database, helps improve patient outcomes by providing 24x7 on call and on-site support and prospectively conducts FDA post-approval studies through the IRB-approved cVAD Study.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA PMA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5™ with Smart Assist® are U.S. FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP® is U.S. FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. Impella is the most studied mechanical circulatory support device in the history of the FDA with real world clinical data on more than 100,000 patients and more than 550 peer-reviewed publications.
In Europe, the Impella 2.5, Impella CP and Impella CP with SmartAssist are CE marked for treatment of high-risk PCI and AMI cardiogenic shock patients for up to 5 days. Impella 5.0 and Impella LD are CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 10 days. The Impella 5.5™ with Smart Assist® is CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 30 days. The Impella RP is CE marked to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia.
To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit www.impella.com.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit: www.abiomed.com.
Abiomed, Impella, Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, and Impella Connect are registered trademarks of Abiomed, Inc., and are registered in the U.S. and certain foreign countries. Impella BTR, Impella 5.5, Impella ECP, CVAD Study, and SmartAssist are pending trademarks of Abiomed, Inc. Other trademarks and service marks appearing in this document are the property of their respective holders.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of Abiomed's existing and new products, the company's progress toward commercial growth, and future opportunities and expected regulatory approvals. The company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including uncertainties associated with development, testing and related regulatory approvals, including the potential for future losses, complex manufacturing, high quality requirements, dependence on limited sources of supply, competition, technological change, government regulation, litigation matters, future capital needs and uncertainty of additional financing, and other risks and challenges detailed in the company's filings with the Securities and Exchange Commission, including the most recently filed Annual Report on Form 10-K and Quarterly Report on Form 10-Q. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. The company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.
1 Davila, et al., Journal of the American College of Cardiology, Vol. 74, No. 13, Suppl B, 2019
2 M. Basir, Transcatheter Cardiovascular Therapeutics, 2019, Impact of Right Ventricular Dysfunction in AMICS: Insights from the National Cardiogenic Shock Initiative
3 Data on file. cVAD Study. Danvers, MA: Abiomed, January 2014 through June 2019
View source version on businesswire.com: https://www.businesswire.com/news/home/20191025005191/en/
Contacts:
Director, Communications and Public Relations
978-882-8408
tlangford@abiomed.com














