LAS VEGAS - March 1, 2022 - (Newswire.com)
Gb Sciences, Inc. (OTCQB:GBLX), a leading plant-inspired, biopharmaceutical research and development company, has been issued a new patent today by the U.S. Patent and Trademark Office (USPTO) for a cannabinoid-containing mixture designed to treat cardiac hypertrophy, often present in advanced heart disease. Gb Sciences' newly issued patent also covers the use of these receptor-targeted formulations for the treatment of TRPV1-receptor-associated hearing loss and urinary cystitis.
Despite multiple categories of prescription heart medications on the market, heart disease remains the leading cause of death in the United States for people of most racial and ethnic groups. Alternative therapeutic approaches are still needed, especially for the treatment of advanced heart disease. The market for prescription heart disease medications is predicted to rise to $64 billion dollars in the US by 2026, with future market growth fueled by innovative new therapeutic approaches.
"At Gb Sciences, our novel approach to drug discovery combines artificial intelligence and the power of plants to find new treatments based on traditional medicine from around the world," said Dr. Andrea Small-Howard, President, Chief Science Officer, and Director of Gb Sciences. "Our latest patent-protected formulations will potentially help those who are afflicted with advanced heart disease, and they may be useful for the prevention of TRPV1-associated hearing loss and urinary cystitis because of their activity at that important receptor."
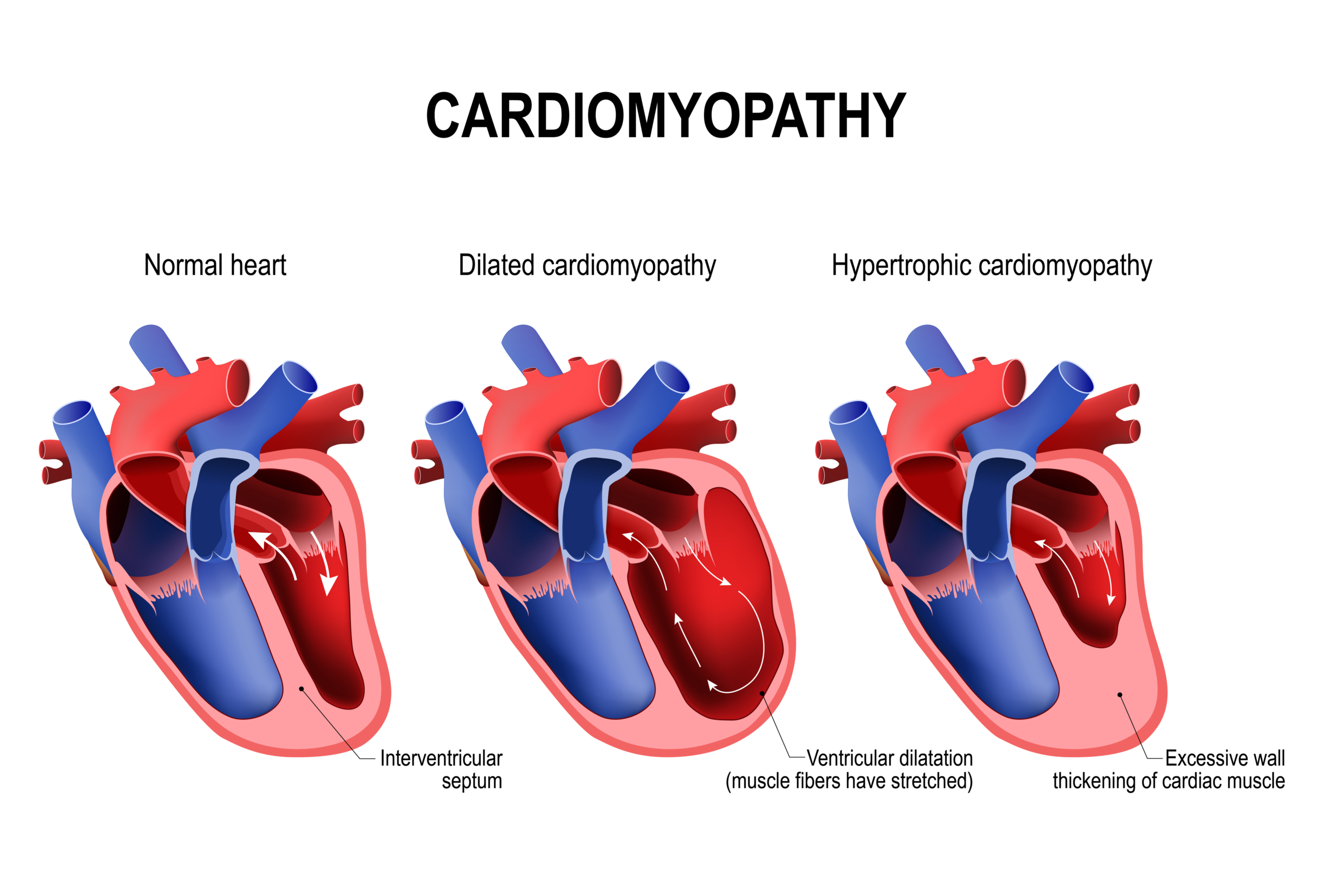 Gb Sciences is committed to developing cannabis- and plant-inspired therapies for the prescription drug market that treat advanced heart disease. Gb Sciences' patent covers methods of treatment using optimized mixtures of cannabis-derived compounds as the Active Pharmaceutical Ingredient ("API"), regardless of whether the individual compounds are derived from the cannabis plant, another plant, synthetically produced, or derived from a combination of sources. Gb Sciences' formulations will be produced using synthetically manufactured molecules that are exact copies of the plant-based ingredients that inspired these formulations, but without the need to grow plants to obtain these ingredients.
Gb Sciences is committed to developing cannabis- and plant-inspired therapies for the prescription drug market that treat advanced heart disease. Gb Sciences' patent covers methods of treatment using optimized mixtures of cannabis-derived compounds as the Active Pharmaceutical Ingredient ("API"), regardless of whether the individual compounds are derived from the cannabis plant, another plant, synthetically produced, or derived from a combination of sources. Gb Sciences' formulations will be produced using synthetically manufactured molecules that are exact copies of the plant-based ingredients that inspired these formulations, but without the need to grow plants to obtain these ingredients.
Currently, Gb Sciences has been issued four U.S. patents for the therapeutic use of plant-derived mixtures. These minimum essential mixtures act as therapeutic agents for specific disease categories including Parkinson's disease, chronic pain, TRPV1-receptor-related disorders, and the orphan-disease, Mast Cell Activation Syndrome (MCAS). Gb Sciences also licenses two issued U.S. patents that cover methods of treating TRPV1-receptor-related disorders. Gb Sciences has three issued patents outside the U.S. and many pending patent applications in the U.S. and internationally. Gb Sciences' novel PhAROS™ drug discovery platform is also patent pending.
To learn more about Gb Sciences, visit www.gbsciences.com.
About Gb Sciences and GbS Global Biopharma
Gb Sciences, Inc. is a plant-inspired biopharmaceutical research and development company creating patented, disease-targeted formulations of plant-inspired therapeutic mixtures for the prescription drug market through its Canadian subsidiary, GbS Global Biopharma, Inc. The "plant-inspired" active ingredients in its therapeutic mixtures are synthetic homologues identical to the original plant compounds but produced under current Good Manufacturing Practices. Gb Sciences' intellectual property portfolio contains six issued U.S. and three issued foreign patents, as well as 18 U.S. and 49 foreign patent-pending applications. In its drug development pipeline, Gb Sciences has five preclinical phase product development programs. Gb Sciences' lead program for Parkinson's disease is being prepared for a first-in-human clinical trial. Gb Sciences' formulations for chronic pain, anxiety, and depression are currently in preclinical animal studies with researchers at the National Research Council Canada. The company also recently received positive preclinical proof-of-concept data supporting its complex mixtures for the treatment of Cytokine Release Syndrome related to COVID-19, and its lead candidates will be optimized based on late-stage preclinical studies at Michigan State University. Gb Sciences' productive research and development network includes distinguished universities, hospitals, and Contract Research Organizations. To learn more, visit www.gbsciences.com.
Forward-Looking Statements
This press release may contain statements relating to future results or events, which are forward-looking statements. Words such as "expects," "intends," "plans," "may," "could," "should," "anticipates," "likely," "believes" and words of similar import may identify forward-looking statements. These statements are not historical facts, but instead represent only the Company's belief regarding future events, many of which, by their nature, are inherently uncertain and outside of the Company's control. It is possible that the Company's actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements. Further, information concerning the Company and its business, including factors that potentially could materially affect the Company's business and financial and other results, are contained in the Company's filings with the Securities and Exchange Commission, available at www.sec.gov. All forward-looking statements included in this press release are made only as of the date of this press release, and we do not undertake any obligation to publicly update or correct any forward-looking statements to reflect events or circumstances that subsequently occur or of which we hereafter become aware.
Media Contact
Savannah Muir
savannah@newswire.com
Press Release Service by Newswire.com
Original Source: Gb Sciences Granted U.S. Patent for Cannabinoid-Containing Treatment for Advanced Heart Disease, TRPV1-Receptor-Associated Hearing Loss and Urinary Cystitis