Exscientia plc (Nasdaq: EXAI)
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220323005753/en/
(Graphic: Business Wire)
Recent advancements in the Company’s pipeline, collaborations and operations as well as financial results for the fourth quarter and full year 2021 are summarised below. In addition, Exscientia will host a conference call on Thursday, March 24 at 12:30 p.m. GMT / 8:30 a.m. ET to provide an overview of the company's end-to-end technology platform and progress towards automating drug discovery and development.
Recent Highlights
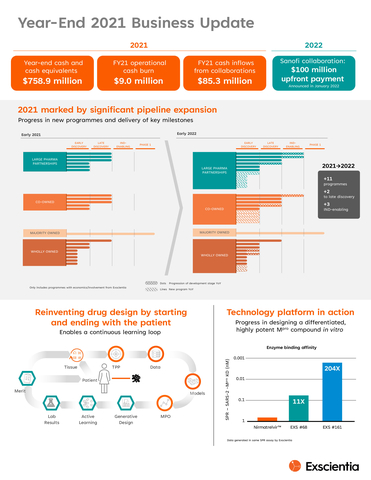
Progressed pipeline led by significant collaborations, expansion, and programme advancement
- Year-over-year pipeline progress with 11 new programmes added, including advancement of three programmes into IND-enabling studies and two programmes to late discovery
- Partnered programmes
- Established collaboration with Sanofi in January 2022 focused on development of up to 15 novel small molecule candidates across oncology and immunology, received $100 million upfront cash payment with the potential of $5.2 billion in total milestones plus tiered royalties; several targets have been identified since signing
- Commenced eighth drug discovery project with Bristol Myers Squibb (BMS) in early 2022 for programme against an undisclosed oncology target
- Achieved preclinical proof of concept milestone for a target within Bayer collaboration supporting advancement into late discovery
- Co-owned programmes
- Entered into IND-enabling studies for GTAEXS-617, a CDK7 inhibitor co-owned with GT Apeiron; IND/CTA submission expected by year-end 2022; additional translational data in several tumour types to be presented at the April 2022 American Association of Cancer Research (AACR) Annual Meeting and throughout 2022
- Oncology target selected with EQRx, the third in the multi-target collaboration
- Wholly and majority owned programmes
- Phase 1 healthy volunteer top-line data for EXS-21546, A2a antagonist in high adenosine signature cancers, on track for the first half of 2022; translational research on identifying adenosine specific gene signatures for patient selection to be presented at AACR 2022
- With support from the Bill & Melinda Gates Foundation, Exscientia has designed an Mpro molecule with pan-coronavirus activity demonstrating 200x greater potency in vitro than a commercially available oral COVID-19 antiviral; ongoing compound development with candidate nomination anticipated in the second half of 2022
Balanced business model has generated meaningful cash flows and strong balance sheet
- $85.3 million cash flow from collaborations in full-year 2021, ending 2021 with $758.9 million in cash and cash equivalents
- 2021 net cash outflows from operations of approximately $9.0 million
- $100 million upfront payment from Sanofi collaboration signed in January 2022
Peer-reviewed publications and upcoming scientific meeting presence showcase impact of translational research, clinical impact of precision medicine platform and differentiated technology platform
- Three abstracts accepted for poster presentation at AACR 2022 highlight the potential of Exscientia's precision medicine platform and AI capabilities in designing optimised molecules, improving translational research and identifying novel pathways
- EXALT-1 clinical trial results published in Cancer Discovery demonstrate the benefit of the first AI-supported functional precision medicine platform to guide treatment selection and improve outcomes in patients with advanced haematological cancers
- Select peer-reviewed publications describe recent advancements in Exscientia's technology platform
- Fragment Hotspot Mapping to Identify Selectivity-Determining Regions Between Related Proteins describes a computational method to map protein binding pockets and identify critical regions that can be exploited to generate selective molecules
- Deep generative design with 3D pharmacophoric constraints demonstrates the potential of a new method (DEVELOP) to utilise 3D representations of molecules and generate compounds with improved properties over previous methods
- Generating property-matched decoy molecules using deep learning showcases a deep learning method for generating molecules to validate and improve virtual screening methods
Strengthened team to position Exscientia for future growth
- Richard Law, D. Phil, has been promoted to Chief Business Officer, effective March 4, with continued focus on driving impactful collaborations across our business models
- Professor Charlotte Deane, Ph.D, joined as Chief Scientist of Biologics AI, from the University of Oxford, focused on the application of AI, machine learning, and the design of protein structures in the discovery and development of novel drug candidates
- Significant progress in building out clinical organisation, including clinical operations, to prepare for pipeline expansion and future clinical trials
- Experienced drug discovery team established in Boston, Massachusetts, to focus on wholly owned pipeline
“2021 was a transformational year for Exscientia as we further validated and built-out our differentiated end-to-end AI platform. We executed across our business as shown by new and expanded collaborations, growing our pipeline with eleven new programmes. We significantly expanded our global operations and scaled our capacity. On top of these achievements, we also completed our upsized IPO on Nasdaq,” said Andrew Hopkins, DPhil., Exscientia’s founder and CEO. “As we look to the future, we believe we are reaching a tipping point for realising the full potential of AI-driven drug creation. We’ve already seen historic investment across the industry. Exscientia has achieved many of the “firsts” in this field and is well-positioned to lead the way. The sheer scale that our AI systems lend to scientific discovery make it possible to keep pace with breaking science in a way never before seen. This year we are focused on bringing together the team we have built and the technologies we have invented to advance new medicine programmes toward the clinic, working with our pharma and biopharma collaborators toward audacious goals to tackle rare diseases and solve persistent scientific challenges, and to continue building out our one-of-a-kind precision medicine platform to bring truly personalised medicine to patients."
Investor Call and Webcast Information
Exscientia will host a conference call on March 24 at 12:30 p.m. GMT / 8:30 a.m. ET. A webcast of the live call can be accessed by visiting the “Investors and Media” section of the Company’s website at investors.exscientia.ai. Alternatively, the live conference call can be accessed by dialling +1 (888) 330 3292 (U.S.), +44 203 433 3846 (U.K.), +1 (646) 960 0857 (International) and entering the conference ID: 8333895. A replay will be available for 90 days under "Events and Presentations” in the “Investors and Media” section of the Exscientia website.
Fourth Quarter and Full Year 2021 Financial Results
For the convenience of the reader, the Company has translated pound sterling amounts to U.S. dollars at the rate of £1.000 to $1.350, which was the noon buying rate of the Federal Reserve Bank of New York on December 31, 2021.
Revenue: Revenue for the full year 2021, was $36.9 million, an increase of $23.9 million compared to the full year 2020, primarily due to the achievement of the opt-in milestone on the first candidate in-licensed under Exscientia’s collaboration with BMS.
R&D and cost of drug discovery: Due to various collaboration structures, R&D expenses may be included under multiple accounting line items. The tables below show how these expenses are separated across the accounting categories.
Three months ended December 31, 2021 ($ millions):
|
COGS |
R&D |
Share of
|
Total |
||||
Partnered Programmes |
6.5 |
- |
- |
6.5 |
||||
Co-owned Programmes |
- |
2.5 |
0.2 |
2.7 |
||||
Internal Pipeline and Technology Development |
- |
22.8 |
- |
22.8 |
||||
Total |
6.5 |
25.3 |
0.2 |
32.0 |
Twelve months ended December 31, 2021 ($ millions):
|
COGS |
R&D |
Share of
|
Total |
||||
Partnered Programmes |
23.1 |
- |
- |
23.1 |
||||
Co-owned Programmes |
- |
4.2 |
1.6 |
5.8 |
||||
Internal Pipeline and Technology Development |
- |
55.3 |
- |
55.3 |
||||
Total |
23.1 |
59.5 |
1.6 |
84.1 |
General and administrative expenses: G&A expenses for the three months ended December 31, 2021, were $8.6 million, or 21.3% of total operating expenses. For the full year 2021, G&A expenses grew by $26.9 million compared to the full year 2020, primarily associated with both an expansion of internal capabilities as well as transaction-related professional services.
Cash inflows: For the full year 2021, Exscientia announced three new partnerships and received $85.3 million in cash inflows from its collaborations.
Cash and cash equivalents: Cash and cash equivalents as of December 31, 2021 were $758.9 million.
SELECTED CONSOLIDATED STATEMENT OF OPERATIONS, CONSTANT CURRENCY CONVERSION (unaudited)
($ millions, except per share data, at the rate of £1.000 to $1.350)
|
Three months ended
|
Twelve months ended
|
||
|
2021 |
2020 |
2021 |
2020 |
Revenue |
5.6 |
5.3 |
37.0 |
13.1 |
Cost of sales |
(6.5) |
(5.4) |
(23.1) |
(19.2) |
Research and development expenses |
(25.3) |
(5.0) |
(59.5) |
(14.7) |
General and administrative expenses |
(8.6) |
(1.9) |
(34.8) |
(7.9) |
Operating expenses |
(40.4) |
(12.4) |
(117.4) |
(41.9) |
Foreign exchange gains/(losses) |
2.9 |
(2.9) |
1.3 |
4.1 |
Other income |
1.0 |
0.5 |
5.1 |
1.6 |
Operating loss |
(30.9) |
(9.5) |
(74.1) |
(31.3) |
Finance income/(expense) |
(0.0) |
(0.0) |
(0.2) |
0.0 |
Share of loss on joint ventures |
(0.2) |
(0.5) |
(1.6) |
(1.6) |
Loss before taxation |
(31.2) |
(9.9) |
(75.9) |
(32.9) |
Income tax benefit |
4.0 |
0.9 |
9.4 |
2.8 |
Loss for the period |
(27.1) |
(9.1) |
(66.5) |
(30.1) |
Net loss per share |
(0.91) |
(0.56) |
(1.33) |
(0.98) |
SELECTED CONSOLIDATED BALANCE SHEET, CONSTANT CURRENCY CONVERSION (unaudited)
($ millions, except per share data, at the rate of £1.000 to $1.350)
|
December 31, 2021 |
December 31, 2020 |
Cash and cash equivalents |
758.9 |
84.5 |
Total assets |
864.9 |
104.9 |
Total equity |
765.2 |
79.0 |
Total liabilities |
99.7 |
25.9 |
Total equity and liabilities |
864.9 |
104.9 |
SELECTED CONSOLIDATED STATEMENT OF CASH FLOWS. CONSTANT CURRENCY CONVERSION (unaudited)
($ millions, except per share data, at the rate of £1.000 to $1.350)
|
December 31, 2021 |
December 31, 2020 |
Net cash flows used in operating activities |
(9.0) |
(28.9) |
Net cash flows used in investing activities |
(35.9) |
(5.1) |
Net cash generated from financing activities |
719.5 |
76.0 |
Net increase in cash and cash equivalents |
674.6 |
42.0 |
About Exscientia
Exscientia is an AI-driven pharmatech company committed to discovering, designing and developing the best possible drugs in the fastest and most effective manner. Exscientia developed the first-ever functional precision oncology platform to successfully guide treatment selection and improve patient outcomes in a prospective interventional clinical study, as well as to progress AI-designed small molecules into the clinical setting. Our pipeline of internal and partnered programmes demonstrates our ability to rapidly translate scientific concepts into precision-designed therapeutic candidates, with more than 25 projects underway. By designing better drugs, faster, we believe the best ideas of science can rapidly become the best medicines for patients.
Exscientia has offices in Oxford, Vienna, Dundee, Miami, Boston, Cambridge (UK) and Osaka. For more information visit us on https://www.exscientia.ai or follow us on Twitter @exscientiaAI.
Forward-Looking Statements
This press release contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including with respect to the progress of discovery and development of candidate molecules, and the timing and progress of, and data reported from, clinical trials of Exscientia’s product candidates, and Exscientia’s expectations regarding its projected revenue and cash runway. Any statement describing Exscientia’s goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to a number of risks, uncertainties and assumptions, including those related to the impact that the COVID-19 pandemic could have on the Company’s business, and including the scope, progress and expansion of Exscientia’s product development efforts; the initiation, scope and progress of Exscientia’s and its partners’ clinical trials and ramifications for the cost thereof; clinical, scientific, regulatory and technical developments; and those inherent in the process of discovering, developing and commercialising product candidates that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such product candidates. In light of these risks and uncertainties, and other risks and uncertainties that are described in the Risk Factors section and other sections of Exscientia’s Registration Statement on Form F-1, filed with the Securities and Exchange Commission (SEC) on September 10, 2021, as amended (File No. 333-259431), and other filings that Exscientia makes with the SEC from time to time (which are available at http://www.sec.gov), the events and circumstances discussed in such forward-looking statements may not occur, and Exscientia’s actual results could differ materially and adversely from those anticipated or implied thereby. Although Exscientia’s forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by the Company. As a result, you are cautioned not to rely on these forward-looking statements.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220323005753/en/
Contacts
Investors:
Sara Sherman
investors@exscientia.ai
Media:
Amanda Galgay
media@exscientia.ai














