PITTSBURGH - March 3, 2020 - (Newswire.com)
Cernostics, a leader in the development of AI-driven image analysis technologies for precision medicine testing, today announced the publication of positive data from a blinded, independent validation study of its breakthrough TissueCypher Barrett’s Esophagus Assay by the American Journal of Gastroenterology.
As the first and only precision medicine test for esophageal pinch biopsies, TissueCypher® is designed, developed, and independently validated to identify patients with Barrett’s esophagus (BE) who are at higher risk of developing esophageal adenocarcinoma (EAC) and may require earlier therapeutic intervention or more frequent endoscopic surveillance. The test has now been validated in six independent clinical studies with leading clinical centers around the world, including Cleveland Clinic, University of Pennsylvania Medical Center, Geisinger Health, and Academic Medical Center in Amsterdam.
“TissueCypher® addresses one of the most pressing unmet needs in our field – the identification of which Barrett’s patients will progress to cancer. A diagnostic test that helps address this problem will be of great use clinically,” says Nicholas J. Shaheen, M.D., MPH, Chief, Division of Gastroenterology and Hepatology, UNC HealthCare, Chapel Hill, NC; Bozymski-Heizer Distinguished Professor of Medicine, University of North Carolina School of Medicine, and current member of the Cernostics’ Medical Advisory Board.
“With its combination of morphology and molecular insights to assess progression risk, TissueCypher addresses a number of challenging issues for GI pathologists. Of most concern is differentiating non-dysplastic BE (NDBE) patients that are histologically identical but progress at significantly different rates to high-grade dysplasia (HGD) or EAC,” says Jon M. Davison, MD, pathologist and co-author of the article and Associate Professor, Department of Pathology, University of Pittsburgh School of Medicine. Davison continues, “This test also adds further prognostic clarity to cases diagnosed as indefinite for dysplasia (IND).”
The peer-reviewed article, written by researchers from the University of Pittsburgh School of Medicine, Cleveland Clinic, and Cernostics, Inc. presents a blinded, independent validation of the TissueCypher® Assay for predicting future development of EAC in patients with BE.
In the study that was funded by the NIH/NCI, patients with BE who progressed to HGD or EAC after at least one year following endoscopy (n=58) were matched to patients with BE without disease progression after a median of seven years’ surveillance (n=210). Baseline biopsies with expert GI pathologist diagnoses of NDBE, IND or LGD were blindly tested and classified by the TissueCypher® Assay into high-risk and low-risk for future progression. Results demonstrated that patients classified as high-risk by TissueCypher® were at 4.7-fold increased risk for HGD/EAC compared to those classified as low-risk group (p<0.0001). In addition, patients with no sign whatsoever of dysplasia, based on expert pathologist review, who scored high-risk by TissueCypher® progressed at a higher rate (26%) than patients with expert pathologist confirmed LGD (21.8%). This is a crucial finding as these are the “at-risk” group who are missed by the current standard of care. A high-risk score with TissueCypher® in patients with non-dysplastic BE may support early use of endoscopic eradication therapy or increased surveillance to prevent development of HGD/EAC.
“This independent validation of TissueCypher® adds to the robust body of clinical evidence supporting this test as an important tool for managing patients with BE,” said Mike Hoerres, CEO, Cernostics.
“TissueCypher® is unique and powerful in that it objectively extracts highly meaningful cellular, molecular and morphologic features from standard pinch biopsies. It fits seamlessly into current clinical care, and no special brushes or alternative esophageal biopsy collection devices are required. So, physicians get an individualized risk score from the biopsies removed during an upper GI endoscopy, which supports clinical decision-making,” said Hoerres.
“The Cernostics product, TissueCypher®, fits seamlessly into a physician’s current clinical practice. No extra steps are needed in the endoscopy suite, as some systems today require,” said Anthony Infantolino, M.D., AGAF, FACG, FACP, gastroenterologist, Professor of Medicine at the Sidney Kimmel Jefferson Medical College and Associate Chairman of the Division of Gi/Hepatology and Director of The Jefferson Barrett’s Center and long-time user of TissueCypher®. “The test evaluates pinch biopsies taken during endoscopy and gives me the adjunctive information I need to determine my patient treatment plan.”
TissueCypher® is commercially available as a laboratory developed test (LDT) through Cernostics’ clinical reference lab, located in Pittsburgh, Pennsylvania.
About Barrett’s Esophagus
BE affects more than three million Americans, occurring when chronic exposure to stomach acid causes the esophageal cell lining to deteriorate and undergo changes that can create an environment for cancer. Without treatment, Barrett’s can lead to EC, with a poor five-year survival of less than 20%. Today, Barrett’s is commonly managed by surveillance, involving regular endoscopic procedures with biopsy, monitoring disease progression, and GERD-related drug therapy to control symptoms and prevent esophageal injury.
About TissueCypher Barrett’s Esophagus Assay
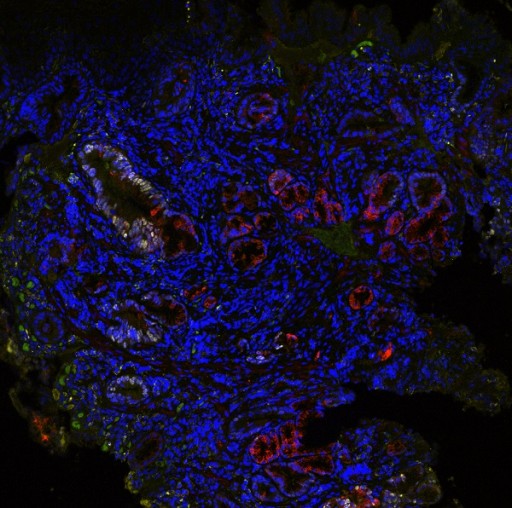
TissueCypher® is a laboratory developed test (LDT) provided as a testing service by Cernostics clinical reference laboratory in Pittsburgh, PA. The test is indicated for evaluation of esophageal pinch biopsies (or Endoscopic Mucosal Resection [EMR] specimens) from patients confirmed to have Barrett’s esophagus with histology of no dysplasia (ND), indefinite for dysplasia (IND) or low- grade dysplasia (LGD). The TissueCypher platform utilizes a multiplexed fluorescence imaging platform that rapidly extracts high dimensional, quantitative data on multiple epithelial, stromal and morphometric biomarkers in biopsies and assess multiple pathways associated with malignant progression.
Experience TissueCypher for yourself by visiting www.ExperienceTissueCypher.com and choosing one of several charts to find a patient comparable to one in your own Barrett’s pool and see how TissueCypher® provides adjunctive information to support your clinical decisions.
About Cernostics
Cernostics, a leader in tissue-based diagnostic testing, provides diagnostic tests with deeper tissue insights, better patient outcomes, and lower cost of care. Cernostics’ mission is to quantify the tissue system complexity, providing physicians and patients with individualized, actionable information to improve outcomes and reduce the incidence and mortality of cancer. For a complete listing of Cernostics’ published patents, visit www.cernostics.com/IP.
Media Contact:
Lisa Bichsel
Cernostics
Tel: +1 719-640-5640
Email: lbichsel@cernostics.com
Related Links
Experience TissueCypher
Press Release Service by Newswire.com
Original Source: Cernostics Announces Groundbreaking Data Demonstrating TissueCypher Performance for Predicting Risk of Progression to EAC in Patients With Non-Dysplastic BE













