Now the only microneedling device FDA-cleared for use on both the face and abdomen
Milestone achievement in just five months since BeautyHealth’s acquisition of the brand
The Beauty Health Company (NASDAQ:SKIN), home to hero brand Hydrafacial, today announced the U.S. Food and Drug Administration (FDA) has cleared its SkinStylus™ microneedling device for use on facial acne scarring in Fitzpatrick skin types I, II, and III in patients aged 22 years and older, making it the only microneedling device FDA cleared for both the face and abdomen.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230802207816/en/
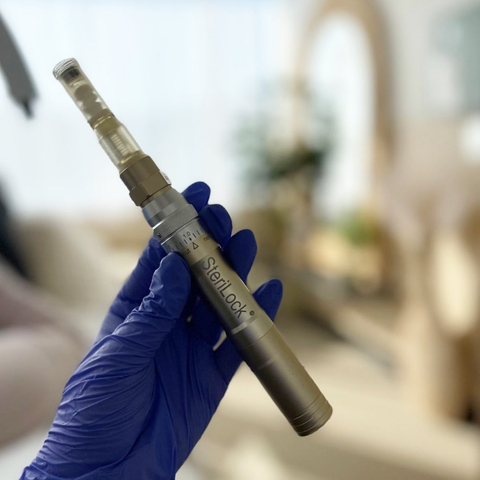
SkinStylus™ (Photo: Business Wire)
The clearance from the FDA is a notable milestone for SkinStylus™ since its acquisition by BeautyHealth in February of this year, revealing of an ambitious and accelerated path for the brand since coming under BeautyHealth’s leadership.
“SkinStylus’ new facial indication for acne scarring is a testament to BeautyHealth’s innovation track record, proven brand building expertise, and our commitment to creating the future of skin health,” said BeautyHealth President and Chief Executive Officer Andrew Stanleick. “Our goal is to become the world’s leading beauty, health and wellness platform by bringing together incredible brands and growing them into category leaders. This SkinStylus indication further unlocks the potential of the highly complementary treatments in our multi-brand ecosystem and positions SkinStylus for exponential growth in the years ahead.”
Expected to reach $1 billion U.S. market size by 20301, microneedling is a minimally invasive treatment that is quickly becoming a favorite amongst providers and skincare lovers. Google searches for microneedling are up +20% YoY and up +88% from five years ago2; and the hashtag term #microneedling has over 4.4 million uses on Instagram3.
Microneedling uses small needles to create tiny, controlled micro injuries that help trigger collagen and elastin production, which is essential for smoother, firmer, and more even-toned skin.
SkinStylus™ is an esthetician-founded brand, and the SkinStylus™ SteriLock® MicroSystem is designed to fit into the way medical and aesthetic professionals work, with a combined feature set they won’t find anywhere else.
Categorized by the FDA as a Class II Medical Device and under its new clearance, SkinStylus™ SteriLock® MicroSystem is intended to be used as a microneedling treatment to improve the appearance of facial acne scars in Fitzpatrick skin types I, II, and III in patients aged 22 years and older.
SkinStylus™ is already FDA-cleared for the indication to improve the appearance of surgical or traumatic hypertrophic scars on the abdomen in adults aged 22 years and older. This was supported by the clinical data that was submitted to FDA during the clearance process.
SkinStylus™ is available at providers across the United States. Learn more by visiting SkinStylus.com.
About The Beauty Health Company
The Beauty Health Company (NASDAQ: SKIN) is a global category-creating company delivering millions of skin health experiences every year that help consumers reinvent their relationship with their skin, bodies, and self-confidence. Our brands are pioneers: Hydrafacial™ in hydradermabrasion, SkinStylus™ in microneedling, and Keravive™ in scalp health. Together, with our powerful community of estheticians, partners, and consumers, we are personalizing skin health for all ages, genders, skin tones, and skin types in more than 90 countries. We are committed to being ever more mindful in how we conduct our business to positively impact our communities and the planet. Find a local provider at https://hydrafacial.com/find-a-provider/, and learn more at beautyhealth.com or LinkedIn.
Forward-Looking Statements
Certain statements made in this release, including statements regarding the potential future market size for microneedling, are “forward looking statements” within the meaning of the “safe harbor” provisions of the United States Private Securities Litigation Reform Act of 1995. When used in this press release, the words “estimates,” “projected,” “expects,” “anticipates,” “forecasts,” “plans,” “intends,” “believes,” “seeks,” “may,” “will,” “should,” “future,” “propose” and variations of these words or similar expressions (or the negative versions of such words or expressions) are intended to identify forward-looking statements.
These forward-looking statements are not guarantees of future performance, conditions, or results, and involve a number of known and unknown risks, uncertainties, assumptions, and other important factors, many of which are outside The Beauty Health Company’s control, that could cause actual results or outcomes to differ materially from those discussed in the forward-looking statements.
Important factors, among others, that may affect actual results or outcomes include The Beauty Health Company’s ability to execute its business plan; market demand for SkinStylus; the ability to effectively provide complementary beauty health products and services; potential litigation involving The Beauty Health Company; changes in applicable laws or regulations; and the possibility that The Beauty Health Company may be adversely affected by other economic, business, and/or competitive factors. The Beauty Health Company does not undertake any obligation to update or revise any forward-looking statements, whether because of new information, future events, or otherwise, except as required by law.
Sources: 1. Emergen Research, Vision Research 2023. 2. Instagram Insights; data pulled as of 7/31/23. 3. Google Analytics data pulled as of 7/31/23.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230802207816/en/
Contacts
BeautyHealth Media: Marina Maher Communications | HydraFacial@hellommc.com
BeautyHealth Investors: The One Nine Three Group | BeautyHealthIR@the193.com













