Acquisition Expands Addressable Market Opportunity and Complements Family of Insulin Delivery Offerings
Conference Call Tuesday, December 13, 2022 at 8:00am ET
Tandem Diabetes Care, Inc. (NASDAQ:TNDM), a global insulin delivery and diabetes technology company, today announced that it has entered into a definitive agreement to acquire AMF Medical SA, the privately held Swiss developer of the Sigi™ Patch Pump. The Sigi Patch Pump is under development and not commercially available. It is designed to be an ergonomic, rechargeable patch pump that reduces the burden of managing diabetes through its use of pre-filled insulin cartridges and its compatibility with automated insulin delivery technology.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221213005314/en/
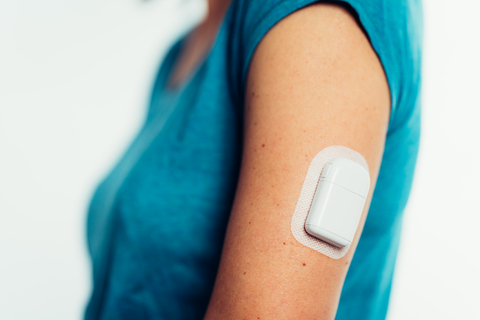
The Sigi™ Patch Pump by AMF Medical. Product is under development and not commercially available. (Photo: Business Wire)
“This acquisition supports our commitment to drive growth through innovation, as AMF Medical has novel technology and a shared passion for creating easy-to-use solutions to improve the lives of people with diabetes,” said John Sheridan, Tandem Diabetes Care president and chief executive officer. “Our portfolio approach to diabetes care is designed to bring the benefits of advanced insulin pump therapy to more people worldwide, utilize our expertise in automated insulin delivery technology and data management, and leverage our commercial infrastructure to create shareholder value.”
Strategic Highlights
AMF Medical’s proprietary and disruptive insulin delivery solution is expected to:
- Expand Tandem’s type 1 and type 2 addressable market opportunities by adding a pump designed for a segment of people living with diabetes not served by Tandem today
- Accelerate time-to-market for a patch pump in Tandem’s portfolio of technology offerings
- Further Tandem’s sustainability objectives by offering an additional pump that minimizes the generation of electronic and battery waste
- Provide a pathway for channel expansion and increased customer access
Terms of the Agreement
Tandem Diabetes Care will acquire AMF Medical under the following financial terms of the agreement:
- A previous strategic investment of Swiss Francs (“CHF”) 8.0 million paid in the third quarter of 2022
- A cash payment of CHF 62.4 million due at closing
-
Additional contingent earnout payments of up to CHF 129.6 million, in aggregate, payable upon the achievement of certain milestones, including:
- Up to CHF 38.4 million upon the successful completion of key development milestones over the next two years
- Up to CHF 91.2 million upon obtaining regulatory clearance of an automated controller enabled (ACE) pump by the United States Food and Drug Administration
The transaction is subject to the satisfaction of customary closing conditions and is expected to close in January 2023.
Baker & McKenzie LLP served as legal advisor to Tandem Diabetes Care, Inc. SVB Securities LLC served as exclusive financial advisor and Homburger AG served as legal advisor to AMF Medical.
Conference Call
The Company will hold a conference call and simultaneous webcast today at 8:00am Eastern Time (5:00am Pacific Time). The link to the webcast is available by accessing the Events & Presentations tab in the Investor Center of the Tandem Diabetes Care website at investor.tandemdiabetes.com, and will be archived for 30 days.
About Tandem Diabetes Care, Inc.
Tandem Diabetes Care, Inc., a global insulin delivery and diabetes technology company based in San Diego, California, creates new possibilities for people living with diabetes, their loved ones, and healthcare providers through a positively different experience. The Company’s human-centered approach to design, development, and support delivers innovative products and services for people who use insulin. Tandem manufactures and sells the t:slim X2 insulin pump with Control-IQ technology. For more information, visit tandemdiabetes.com.
Follow Tandem Diabetes Care on Twitter @tandemdiabetes; use #tslimX2 and #TNDM.
Follow Tandem Diabetes Care on Facebook at facebook.com/TandemDiabetes.
Follow Tandem Diabetes Care on LinkedIn at linkedin.com/company/tandemdiabetes.
© 2022 Tandem Diabetes Care, Inc. All rights reserved. Tandem Diabetes Care, Control-IQ, and t:slim X2 are either registered trademarks or trademarks of Tandem Diabetes Care, Inc. in the United States and/or other countries. All third-party marks are the property of their respective owners.
Forward Looking Statements
This press release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that concern matters that involve risks and uncertainties that could cause actual results to differ materially from those anticipated or projected in the forward-looking statements. These forward-looking statements relate to, among other things, the Company’s future development of new diabetes-related products and services, the Company’s advancement of new insulin pump offerings, including its continued development and introduction of a rechargeable patch pump that features pre-filled insulin cartridges, the ability of the Company to successfully obtain FDA approval for its patch pump, and the expected time-to-market of the patch pump and its ability to influence channel expansion and increase customer access. Additional forward-looking statements relate to the timing and amount of any future earnout payments. These statements are subject to numerous risks and uncertainties, including the Company’s ability to innovate and manage growth, the Company’s ability to successfully integrate AMF Medical’s patch pump products and designs into the Company’s diabetes-related products and services, the Company’s ability to successfully complete the development of the patch pump and related manufacturing processes and secure regulatory approvals for a new patch pump, market acceptance of the Company’s existing products and products under development by physicians and people with diabetes, the potential that newer products, or other technological breakthroughs for the monitoring, treatment or prevention of diabetes, may render the Company’s products obsolete or less desirable, as well as other risks identified in our most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, and other documents that we file with the Securities and Exchange Commission. Our actual results may differ materially from those contemplated by these forward-looking statements. We caution readers to not place undue reliance on these forward-looking statements, which speak only as of the date of this release. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict them all. Tandem undertakes no obligation to update or review any forward-looking statement in this press release because of new information, future events or other factors.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221213005314/en/
Contacts
Media Contact:
858-255-6388
media@tandemdiabetes.com
Investor Contact:
858-366-6900
IR@tandemdiabetes.com













