Preclinical research suggests that restoring lymphatic flow in the brain has the potential to address a range of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, and associated neuroinflammation
Additionally, research found that improving lymphatic flow could enhance the efficacy of antibody therapies against aberrant proteins, potentially opening a new avenue for disease management
PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) (“PureTech” or the “Company”), a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, today announced the publication of preclinical research in Nature which suggests that enhancing meningeal lymphatic drainage could improve clinical outcomes for Alzheimer’s disease, either alone or in combination with passive immunotherapies such as antibodies directed at amyloid beta (Aβ). The paper also uncovered a link between dysfunctional meningeal lymphatics and damaging microglia activation in Alzheimer’s disease, suggesting another route by which restoring healthy drainage patterns could improve clinical outcomes.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210428005790/en/
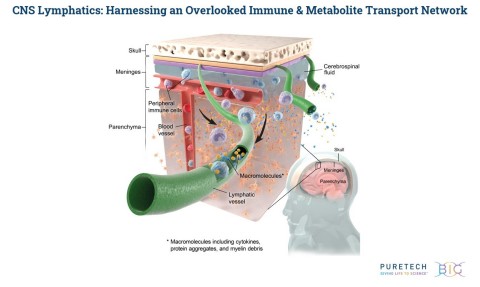
PureTech announced the publication of new research in Nature, which demonstrates that modulation of meningeal lymphatics may help to address Alzheimer’s disease pathologies. PureTech is advancing this work via its wholly-owned meningeal lymphatics research program. (Graphic: Business Wire)
PureTech holds an exclusive license to the underlying intellectual property and is advancing this work via its wholly-owned meningeal lymphatics research program. Through this program, PureTech aims to advance novel therapeutic approaches to modulate lymphatic flow in the central nervous system (CNS) for the potential treatment of a range of neurodegenerative and neuroinflammatory diseases.
This research was led by PureTech collaborator Jonathan Kipnis, Ph.D., the Alan A. and Edith L. Wolff Distinguished Professor of Pathology and Immunology and a BJC Investigator at Washington University School of Medicine in St. Louis, and Sandro Da Mesquita, Ph.D., Assistant Professor of Neuroscience at Mayo Clinic, in collaboration with PureTech scientists. Dr. Kipnis’s lab pioneered the identification, characterization and understanding of the meningeal lymphatics, which connect the brain to the immune system, and has published several groundbreaking papers elucidating the system’s role in clearing waste from the CNS to modulate neurological health. The new paper builds on foundational research published in Nature in 2018, in which Dr. Kipnis’s lab showed that disrupting meningeal lymphatic drainage promoted Aβ accumulation in preclinical models of Alzheimer’s disease. His most recent work finds that improving meningeal lymphatic drainage enhances Aβ clearance in conjunction with passive immunotherapies and modulates microglia activation to improve neural tissue homeostasis in aged transgenic Alzheimer’s disease mice.
“Step by step, we have illuminated the crucial role the meningeal lymphatics system plays in maintaining neurological health. With these recent findings, we have taken an important leap forward by demonstrating the potential to improve clinical outcomes by restoring lymphatic flow in the brain,” Dr. Kipnis said. “These foundational findings in preclinical Alzheimer’s models are exciting because they point the way to potential therapeutics in an area where the need is profound and devastating. I look forward to continuing to collaborate with the team at PureTech as we seek to apply these and other findings to the development of new treatments for neurodegenerative and neuroinflammatory conditions.”
Protein clumping in the brain, including aggregates of Aβ, tau and alpha synuclein, are a well-known hallmark of major neurodegenerative disorders, among them Alzheimer’s disease and Parkinson’s disease. Antibodies have been developed to target these clumps of aberrant proteins, but these treatments historically have shown limited clinical efficacy. Working with transgenic Alzheimer’s disease mice, Dr. Kipnis and his team found that deterioration of lymphatic vascular in the dorsal meninges was correlated with a significant increase in Aβ throughout all analyzed regions of the brain. Further analysis suggested that reduced lymphatic drainage may lead to improper activation of microglia, diminishing their ability to process and present antigens to adaptive immune cells and potentially impairing efficacy of passive immunotherapies such as a Aβ-targeting antibodies. Dr. Kipnis found that Aβ clearance improved markedly when the therapeutic antibodies were injected directly into the cerebrospinal fluid (CSF) of transgenic Alzheimer’s disease mice that had been treated with viral-mediated mVEGF-C to enhance meningeal lymphatic drainage. The new research suggests that improving lymphatic flow could enhance the efficacy of antibody therapies, especially those administered directly to the CSF, opening a new avenue for disease management.
“This new research reinforces our belief that modulating the meningeal lymphatics system may be a key to treating not just Alzheimer’s disease, but other neurological disorders as well,” said Joseph Bolen, Ph.D., Chief Scientific Officer at PureTech Health. “We are proud to be working with Dr. Kipnis, a true scientific pioneer, to advance therapeutic discovery programs leveraging these findings. We hope to make a real difference to patients by bringing these foundational discoveries about brain biology to life.”
About the Meningeal Lymphatics Research Program
PureTech is exploring multiple ways of altering lymphatic flow, both in the CNS and other parts of the body. The CNS was previously thought to be devoid of lymphatic vasculature; however, research published in Nature in 2015 identified the meningeal lymphatic and glymphatic systems as key maintenance organs within the brain. The biological crosstalk at the CNS-immune interface has been further supported by evidence published as the cover story in Nature in 2018, which demonstrated that modulation of lymphatic function in the brain may potentially prevent or delay diseases associated with neurodegeneration, including Alzheimer’s disease, Huntington’s disease and age-associated cognitive decline. This CNS-immune interface has also been supported by evidence published in Nature Neuroscience in 2018, which identifies a direct connection between the CSF and the meningeal lymphatic system, suggesting that modulation of the meningeal lymphatic vessel network has the potential to ameliorate symptoms in CNS disorders, such as multiple sclerosis. These insights underlie PureTech’s approach to designing novel categories of therapeutics to address debilitating and devastating CNS disorders, and the related intellectual property has been exclusively licensed to PureTech.
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, including inflammatory, fibrotic and immunological conditions, intractable cancers, lymphatic and gastrointestinal diseases and neurological and neuropsychological disorders, among others. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders. This pipeline, which is being advanced both internally and through PureTech's Founded Entities, is comprised of 26 therapeutics and therapeutic candidates, including two that have received FDA clearance and European marketing authorization, as of the date of PureTech’s most recently filed Annual Report on Form 20-F. All of the underlying programs and platforms that resulted in this pipeline of therapeutic candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on the Company's unique insights into the biology of the brain, immune and gut, or BIG, systems and the interface between those systems, referred to as the BIG Axis.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements, including statements that relate to the company's future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks and uncertainties that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, our expectations regarding the potential therapeutic benefits of our therapeutic candidates, our expectations regarding the meningeal lymphatics research program and the potential therapeutic benefits based on our published preclinical findings including the potential for treatment in a range of neurodegenerative and neuroinflammatory diseases and those risks and uncertainties described in the risk factors included in the regulatory filings for PureTech Health plc. These forward-looking statements are based on assumptions regarding the present and future business strategies of the company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, neither the company nor any other party intends to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210428005790/en/
Contacts
Investors
Allison Mead Talbot
+1 617 651 3156
amt@puretechhealth.com
U.S. media
Stephanie Simon
+1 617 581 9333
stephanie@tenbridgecommunications.com














